Can Cancer Be Treated by Changing Its Cells?
Tumors grow when cells lose their biological identity. A promising therapeutic might restore their sense of self. Read story
Breast cancer organoids derived from human patients. Breast cancer cells are labeled in purple, DNA is labeled in blue, and a cytoskeleton protein is labeled yellow. Image by Sonam Bhatia and Erika Wee
Long Non-Coding RNAs: From Basic Biology to Therapeutic Targets
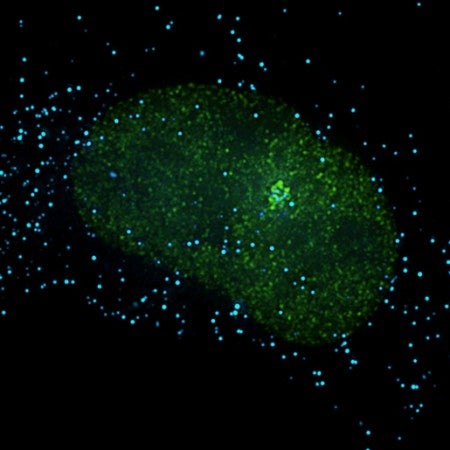
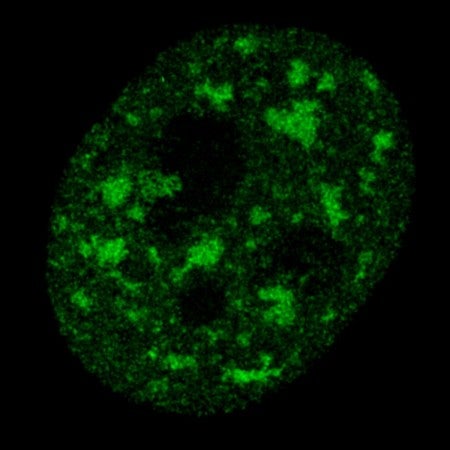
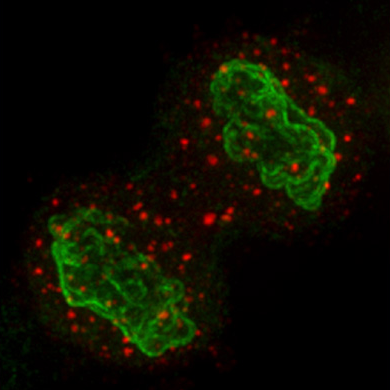
Large-scale genome-wide studies have revealed that only 2% of the human genome encodes for mRNAs that are translated into proteins while as much as 80% of the genome can be transcribed. Of these non-coding RNA transcripts, long non-coding RNAs (lncRNAs) represent the largest and most diverse class. Genome-wide analyses have identified approximately 19,000 human lncRNA genes representing approximately 27% of annotated human genes. As only a relatively small number of these lncRNAs have been functionally characterized and studied in the context of cancer, they represent a unexplored class of nucleic acid regulatory molecules with great potential to impact our insights into cancer biology and treatment. LncRNAs tend to localize to cell nuclei implying roles in gene expression and/or nuclear organization. Our laboratory has played a pivotal role in identifying and characterizing lncRNAs that are involved in breast cancer progression. In addition, we pioneered the use of antisense oligonucleotides (ASOs) to knockdown lncRNAs demonstrating their therapeutic potential.
With breast cancer being the most frequent malignancy in women worldwide, a major focus of our laboratory has been to identify and characterize lncRNAs that play roles in breast cancer progression, and evaluate their mechanism of action and potential as therapeutic targets. Tumor organoids provide a very innovative and unique platform to study cancer, as they can recapitulate many aspects of the disease with high fidelity. As such they represent an excellent system for identifying new therapeutic targets and for drug development and screening in a patient-specific manner. We have developed and characterized a patient-derived breast tumor organoid biobank to identify new therapeutic targets and for drug development and screening in a patient-specific manner. We performed a comprehensive genomic, transcriptomic, and cellular analysis of 87 breast cancer patient-derived organoid (PDO) lines developed in our lab, out of which 50 have been fully validated as being tumor-derived and retain key genomic properties of breast cancers including SNVs and CNAs.
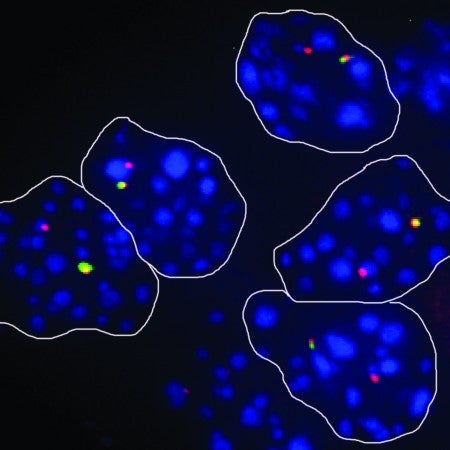
Using our RNA-seq expression data from triple-negative breast cancer (TNBC) and normal PDOs, we performed differential gene expression analysis and we identified ~200 lncRNAs that are upregulated in TNBC PDOs vs normal breast organoids. We cross referenced this list with lncRNAs found in the basal-like cohort of TCGA breast cancer cases, to identify a robust list of intergenic TNBC lncRNA candidates that are the focus of our current studies.